Latest News
Reflections from AACR 2025: AIC Gains International Attention in the Fight Against Myeloma
Conference Observations
After attending the 2025 AACR Meeting (The American Association for Cancer Research), I visited Chicago to attend the AACR Meeting 2025. Despite being late April, Chicago’s weather is chilly. True to its nickname, the Windy City, the wind feels like a sharp, cold blade against the face.
The AACR, the world’s largest cancer research association with over 50,000 members, saw more than 20,000 attendees at this 2025 meeting. According to the association’s CEO, about 700 cancer experts from South Korea, including members of the Korean Cancer Association, attended this year—a slight decrease from last year’s 900.
Founded in 1907, the AACR hosts over 5,000 research presentations in its poster sessions alone. From April 25–30, leading cancer centers like MD Anderson and Moffitt Cancer Center, along with multinational pharmaceutical companies, showcased cutting-edge cancer diagnostics, treatments, and anticancer drugs. It took over 100 years to progress from cytotoxic anticancer drugs to immune checkpoint inhibitors, but what is the 5-year survival rate for late-stage cancer patients?
“Around the turn of the century, many scientists believed a cure for cancer was imminent. Newspapers were filled with claims about causes and cures, but these were quickly debunked or unproven.” — AACR History
Despite over a century of tireless research by countless scientists, we still haven’t conquered cancer.
Twenty years ago, when the human genome map was unveiled, people cheered that cancer was defeated, but the excitement has since faded.
A senior oncologist who has attended the conference for 25 years remarked, “I no longer hold expectations, but don’t we still have to try?” Similarly, an American professor specializing in multiple myeloma asked, “Even with radiation and the pain of chemotherapy or bone marrow transplants, how can we eliminate cancer cells hiding in the bone?”
Perhaps it’s time to explore coexistence with cancer. Instead of using excessive drugs to kill every cancer cell, which can lead to fatal side effects, should we aim to destroy 90% of cancer cells, moderate anticancer drug use, and incorporate broader alternative therapies to live alongside cancer?
Intriguingly, I heard from a Moffitt Cancer Center professor that they’ve allocated $20 million to recruit mathematicians to analyze the relationships between cancer cell counts, growth rates, anticancer drugs, and mortality through mathematical modeling.
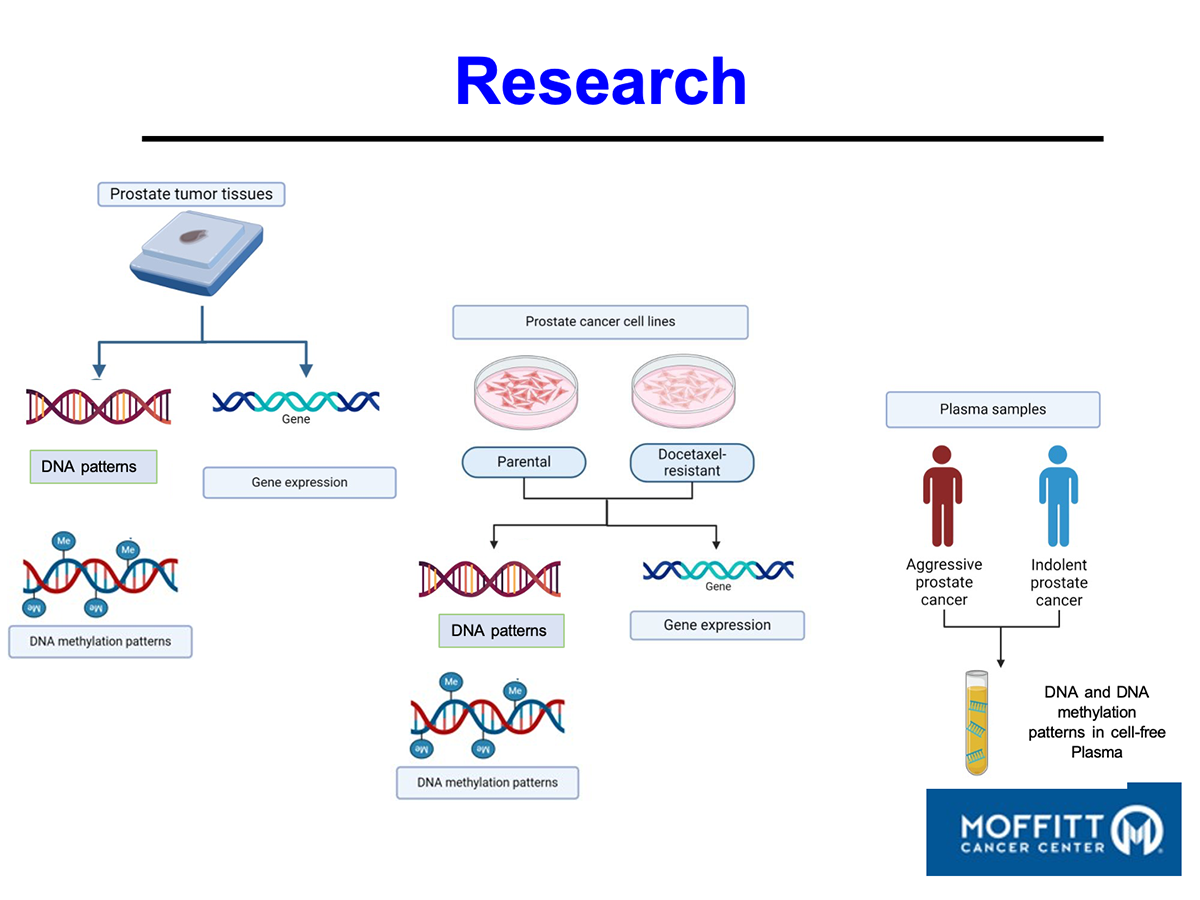
An oncologist from MD Anderson Cancer Center, who visited our booth, expressed great interest in AIC calcium, saying, “It’s the most intriguing substance I’ve come across so far.” The Rockefeller Cancer Center at the University of Arkansas presented the effects of AIC calcium on multiple myeloma during a poster session, details of which are available on the AACR website.
Research Abstract
A Potent Calcium Pretreatment Not Only Induces Bone Formation But Also Prevents Myeloma Progression
Authors
Syed Hassan Mehdi¹, Dongjoon Lee¹, Alex Lee², Paul K. Lee², Donghoon Yoon¹, Jong Park³
¹ Myeloma Center, the University of Arkansas for Medical Sciences, Little Rock, AR, USA
² CBHI (Calcium & Bone Health Institute), Coquitlam, British Columbia, Canada
³ Moffitt Cancer Center, Tampa, FL, USA
Abstract
Multiple myeloma (MM) is the second most common blood cancer. It remains an incurable disease due to relapsing and developing treatment resistance. Treatment resistance and disease progression are not solely due to intrinsic MM characteristics but are also fostered by alterations within the surrounding BM microenvironment. The bone serves as a home for growing myeloma but is also a target organ for myeloma cells. The MM-bone disease (MMBD) is one of the defining features of MM. A previous report showed that myeloma cells colonize the endosteal niche, enter a dormant state, and are activated by bone-lining cells. Many attempts, including ours, were made to test novel or existing bone anabolic drugs for anti-MMBD. They found they did not significantly affect myeloma progression, although bone formation has improved. Interestingly, we found that the surviving mice from myeloma transplants become tolerable to subsequent tumor transplantation. We hypothesized that improving the bone environment may lead to the prevention of myeloma growth. Anti-Orbital Ionic Calcium (AIC) is a chemically/physically modified calcium carbonate with a unique weak bonding and is available as a dietary supplement. It induces bone formation by increasing the ionized calcium in the body. We pretreated mice with AIC for 4 weeks before 5TGM1-transplanted mice (TbT) and then tested myeloma growth. It may mimic a treatment in the pre-emerging or relapsed period of MM. We divided the 8–12-week-old NOD SCID gamma mice into PBS, TbT, and TaT. AIC was gavaged twice daily, five days/week, for four weeks before the transplantation (TbT) or two weeks after transplant (TaT), while PBS was gavaged to the control group when TbT started. The 1×10⁶ luciferase-expressing 5TGM1 cells were injected into the mice via the tail vein. Mice were imaged weekly by IVIS imager to assess the myeloma progression and terminated when endpoint criteria were met. All surviving mice were sacrificed one week after all PBS group mice died.
We found that the median survival of PBS-treated mice was 39 days, while 45 days in the TaT group. To our surprise, >60% of TbT group mice survived until the end of the study. The bioluminescence image analysis showed that myeloma slowly progressed in both treated group mice. DEXA results demonstrated that bone minerals significantly increased in the spine of AIC-treated mice compared to the control mice. Trabecular thickness and bone volume density were significantly higher in the AIC-treated group at the lumbar spine compared to the PBS group.
We also tested 14 PBS-treated or 20 AIC-treated at TbT on 5TGM1-transplanted immune competent C57BL/KaLwRij mice. We found a significant bone matrix increase and improvement of median survival on AIC treatment, with <40% mice lost until 70 days post-transplantation, while 39 days of median survival on PBS-treated, with 80% of mice lost due to MM phenotype.
Conclusion
In summary, we discovered that pretreatment with potent calcium, AIC, significantly prevents MM progression in both immune-deficient and competent mice. We conclude that improving the bone microenvironment with potent calcium pretreatment prevents myeloma cell engraftment and/or growth in mice. These results may suggest a novel therapeutic regimen for MM during pre-emerging or remission phases when treatment safety is ensured.
Clinical Study Demonstrates the Effectiveness of AIC Calcium on Bone Mineral Density: Results from Moffitt Cancer Center
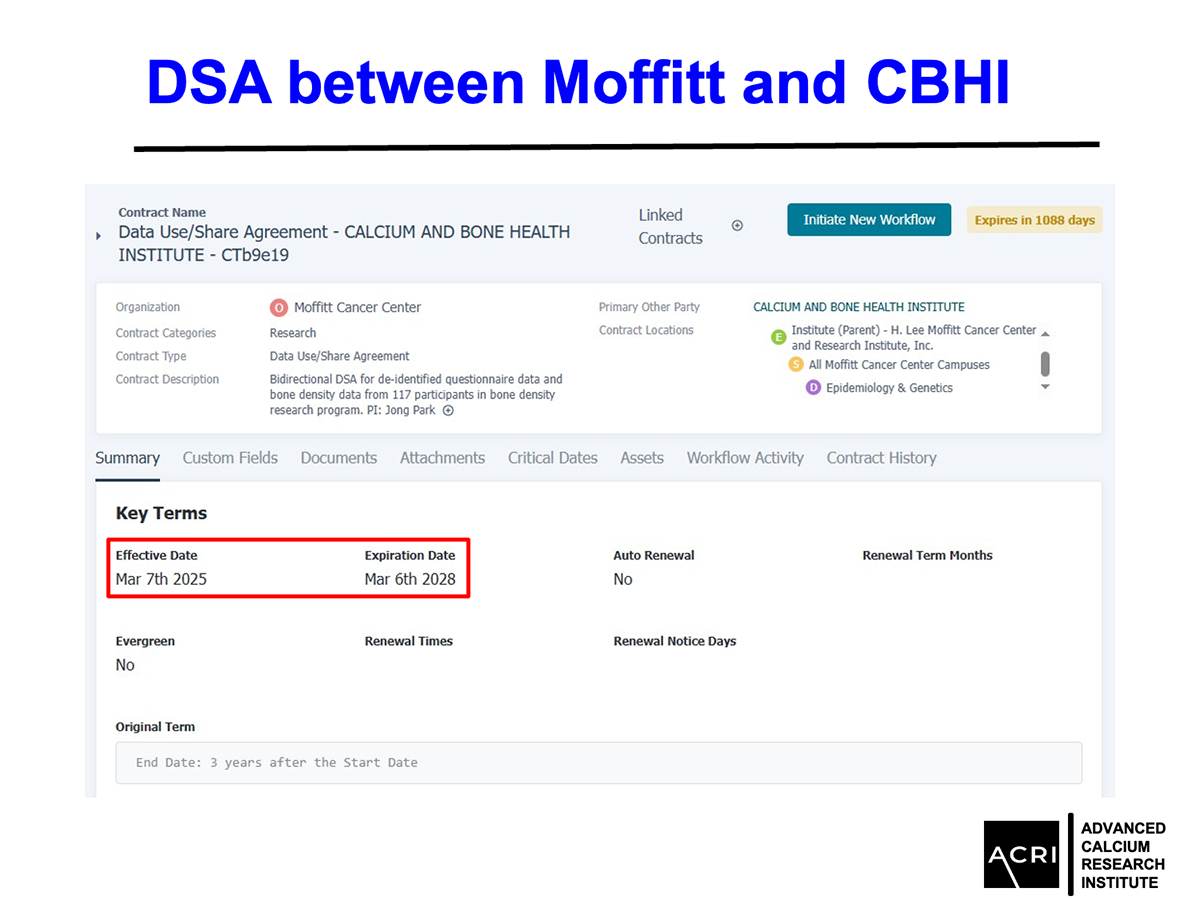
We are pleased to share the results of a collaborative clinical investigation between Moffitt Cancer Center, the Calcium & Bone Health Institute (CBHI) and Advanced Calcium Research Institute (ACRI), examining the impact of Anti-Orbital Ionic Calcium (AIC) on bone mineral density in aging populations.
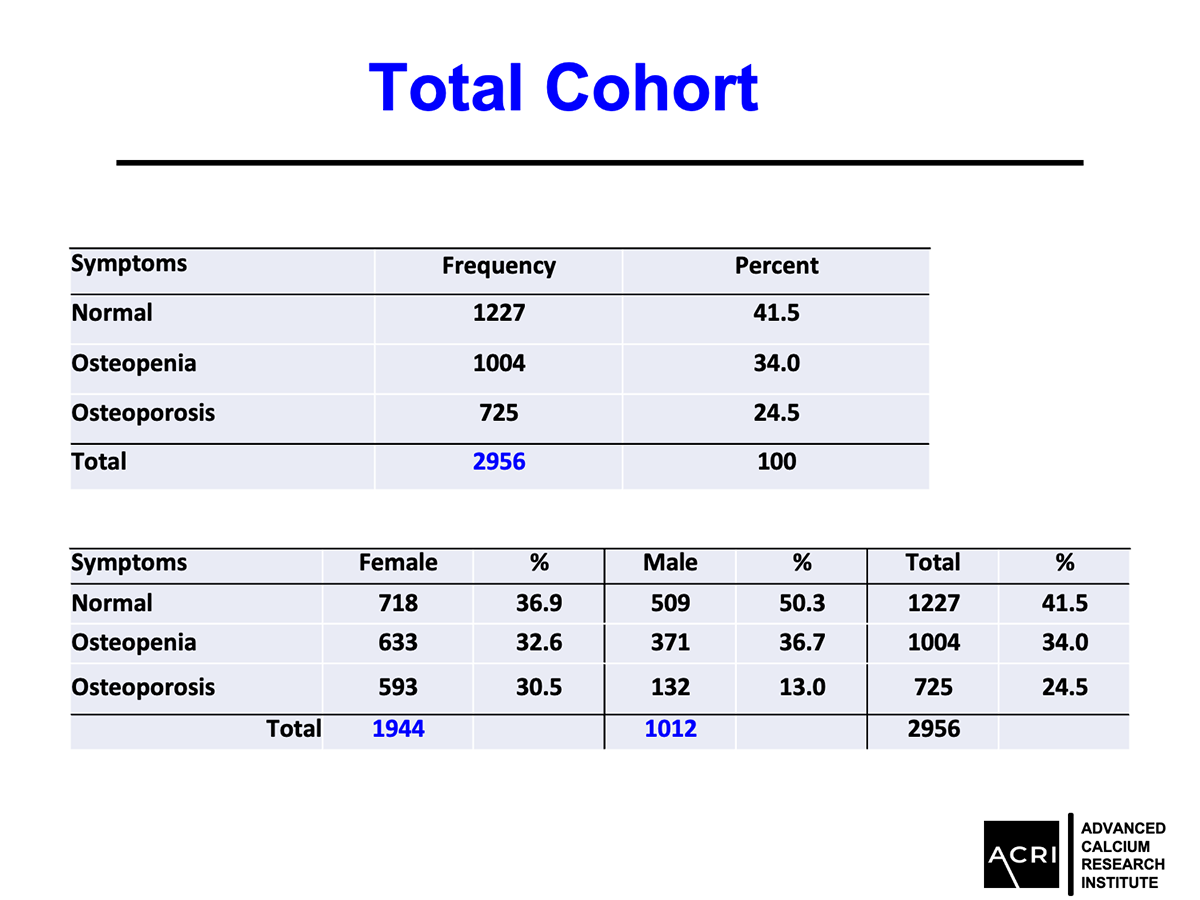
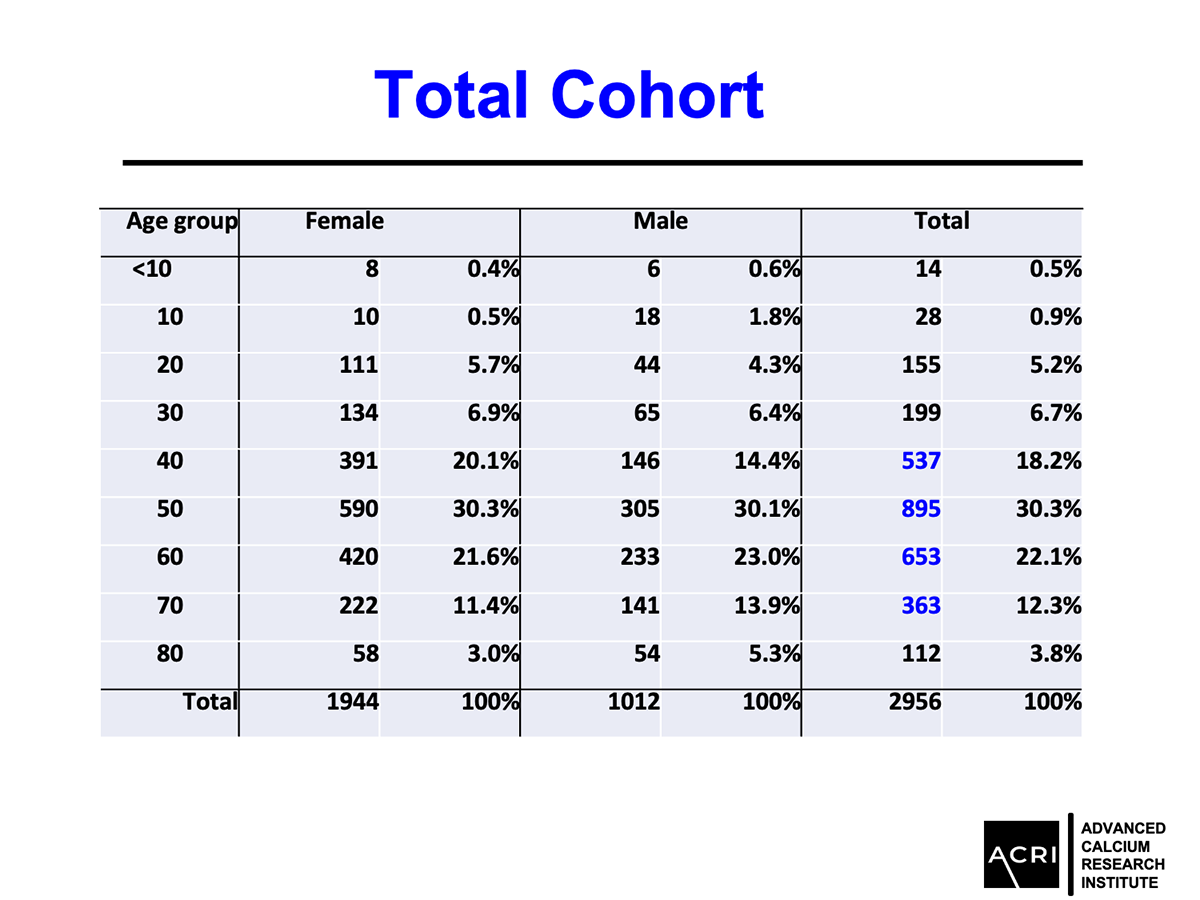
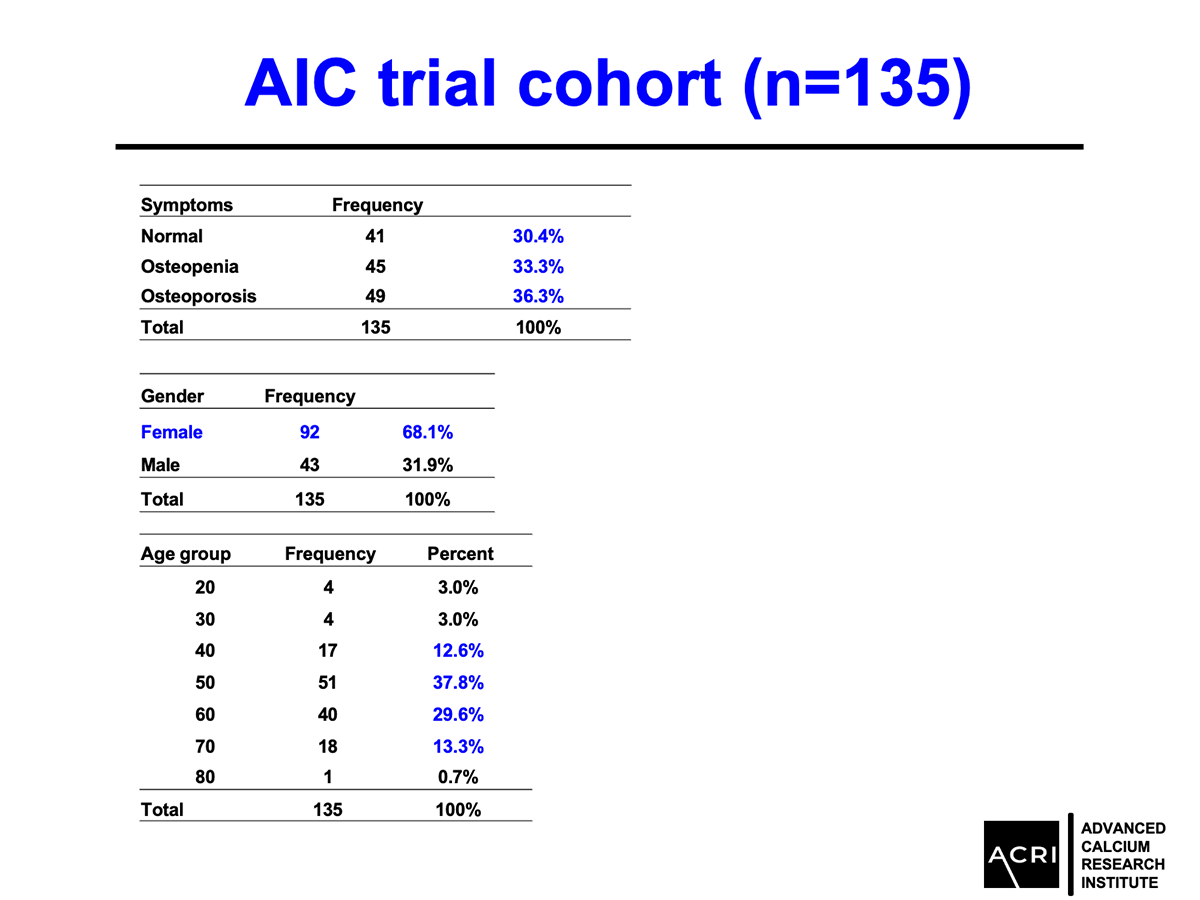
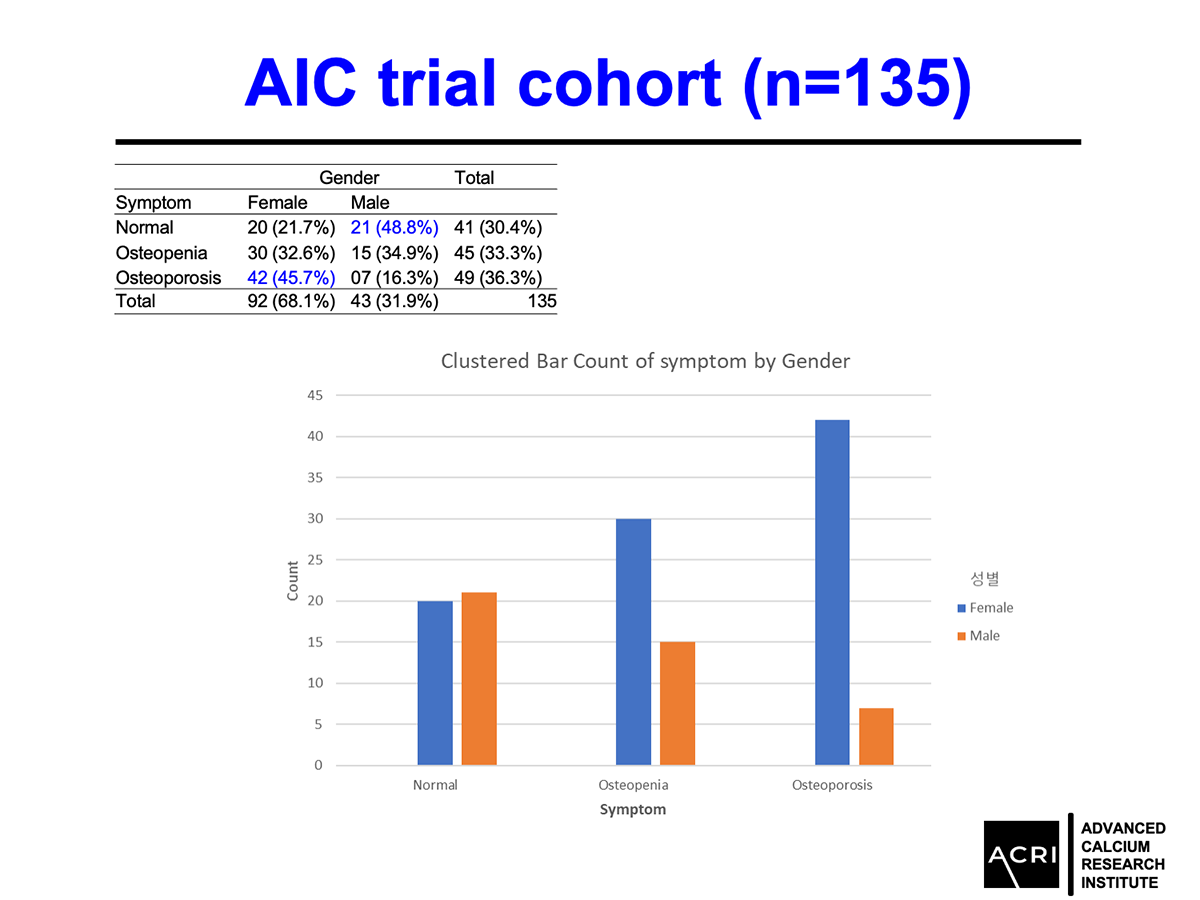
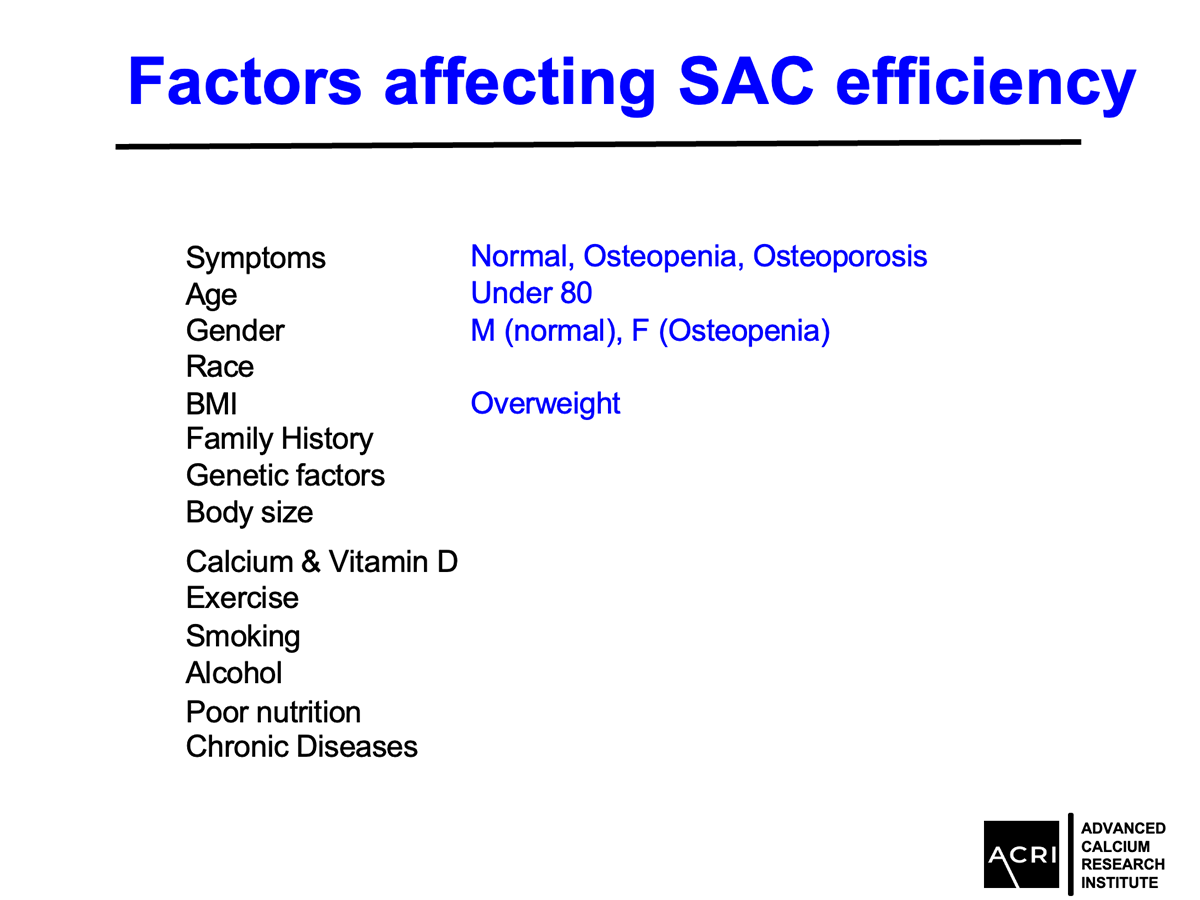
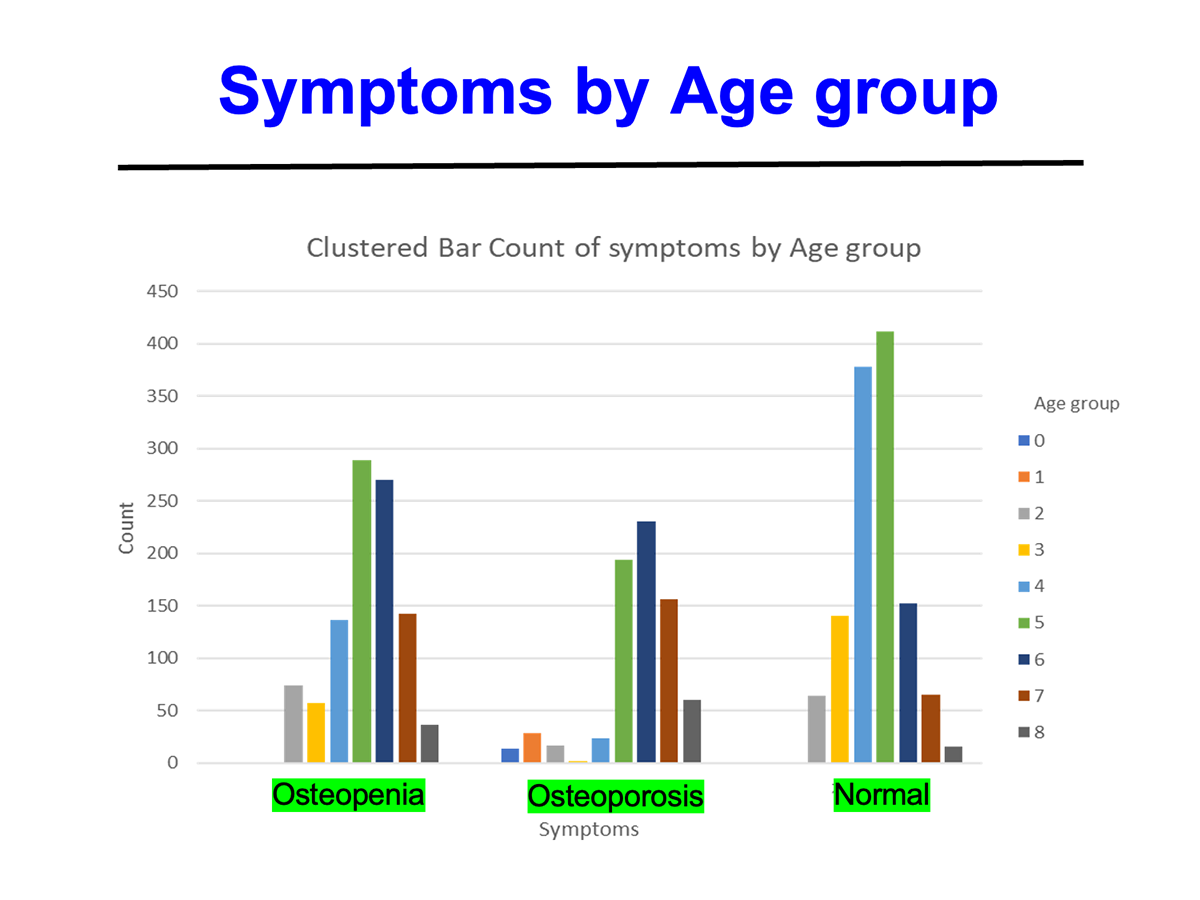
AIC calcium, a formulation designed to deliver bioavailable calcium ions without dependence on stomach acid or vitamin D, was evaluated in a 135-subject clinical cohort with varying degrees of bone health status—ranging from normal to osteopenia and osteoporosis. Key findings include:
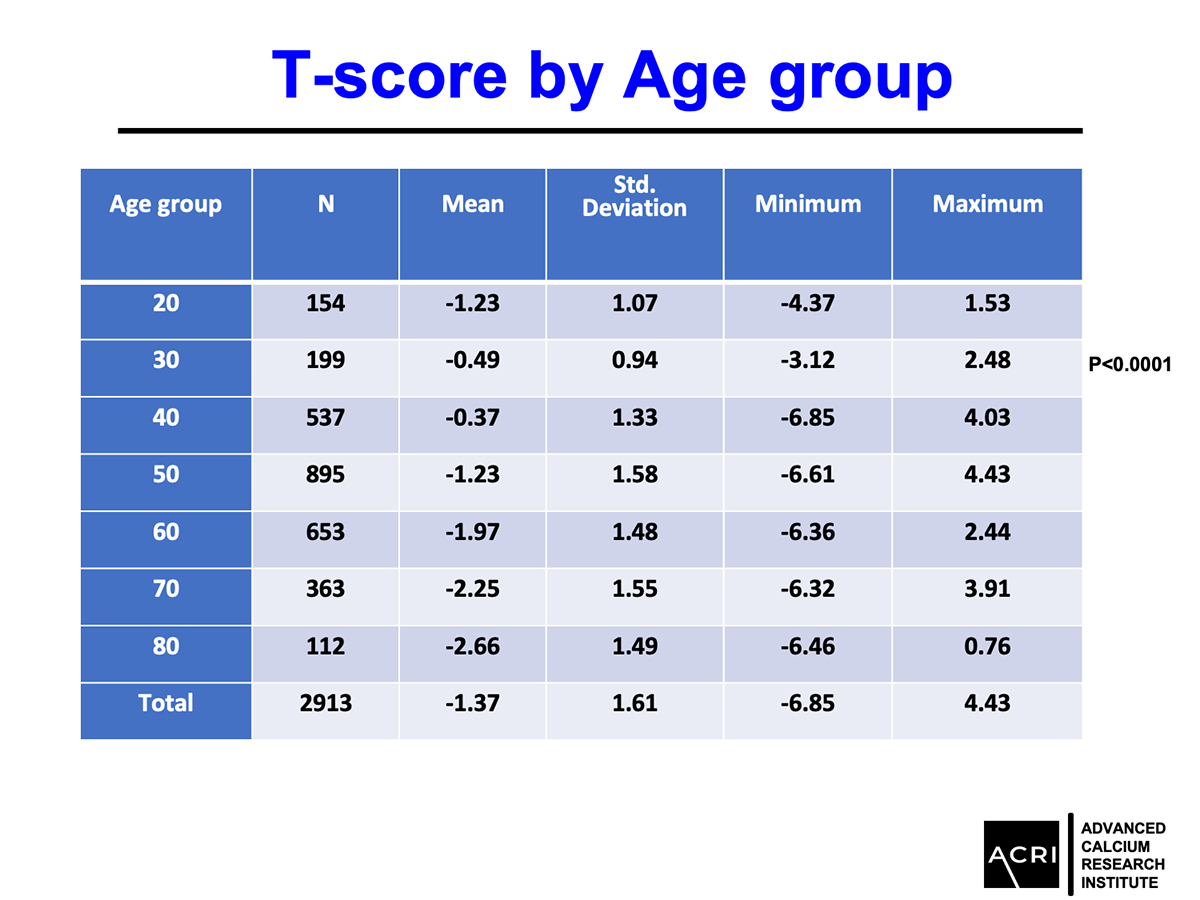
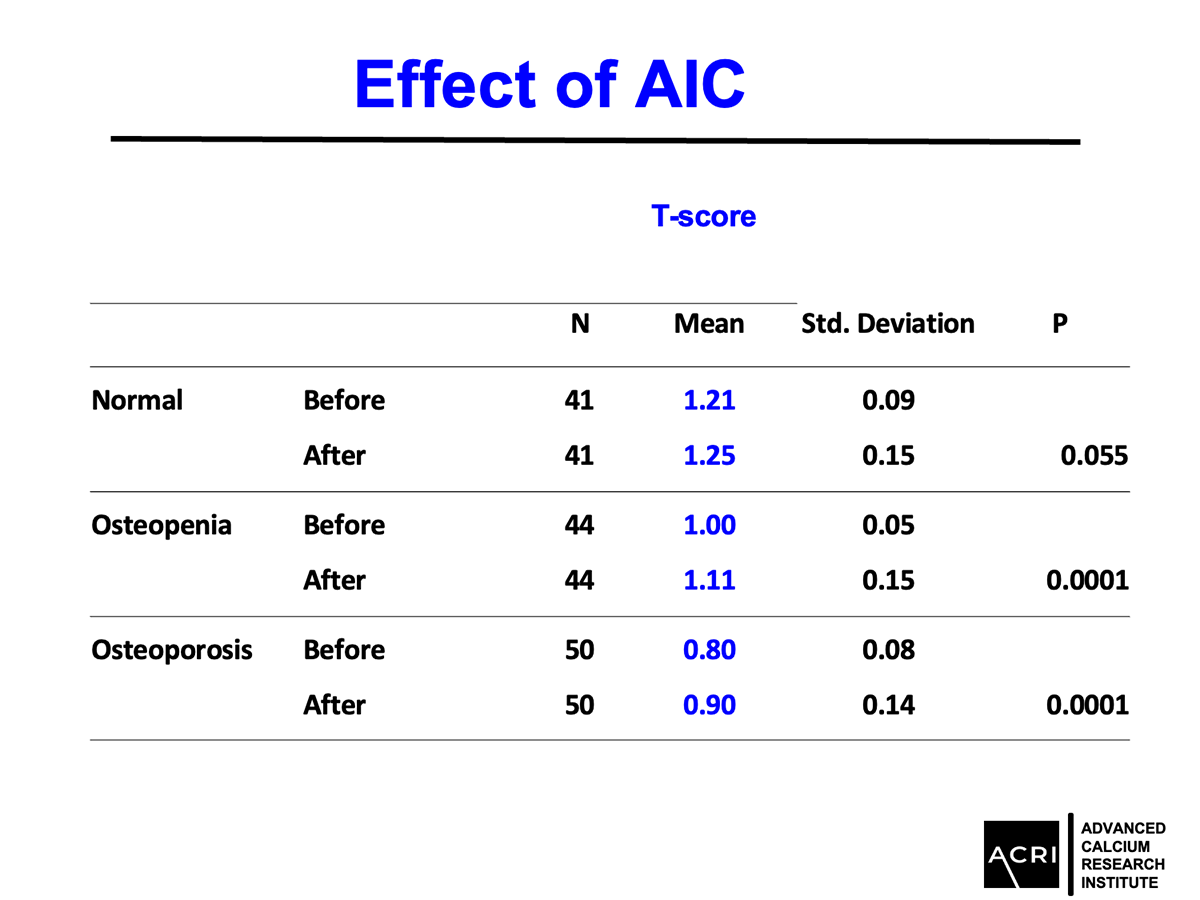
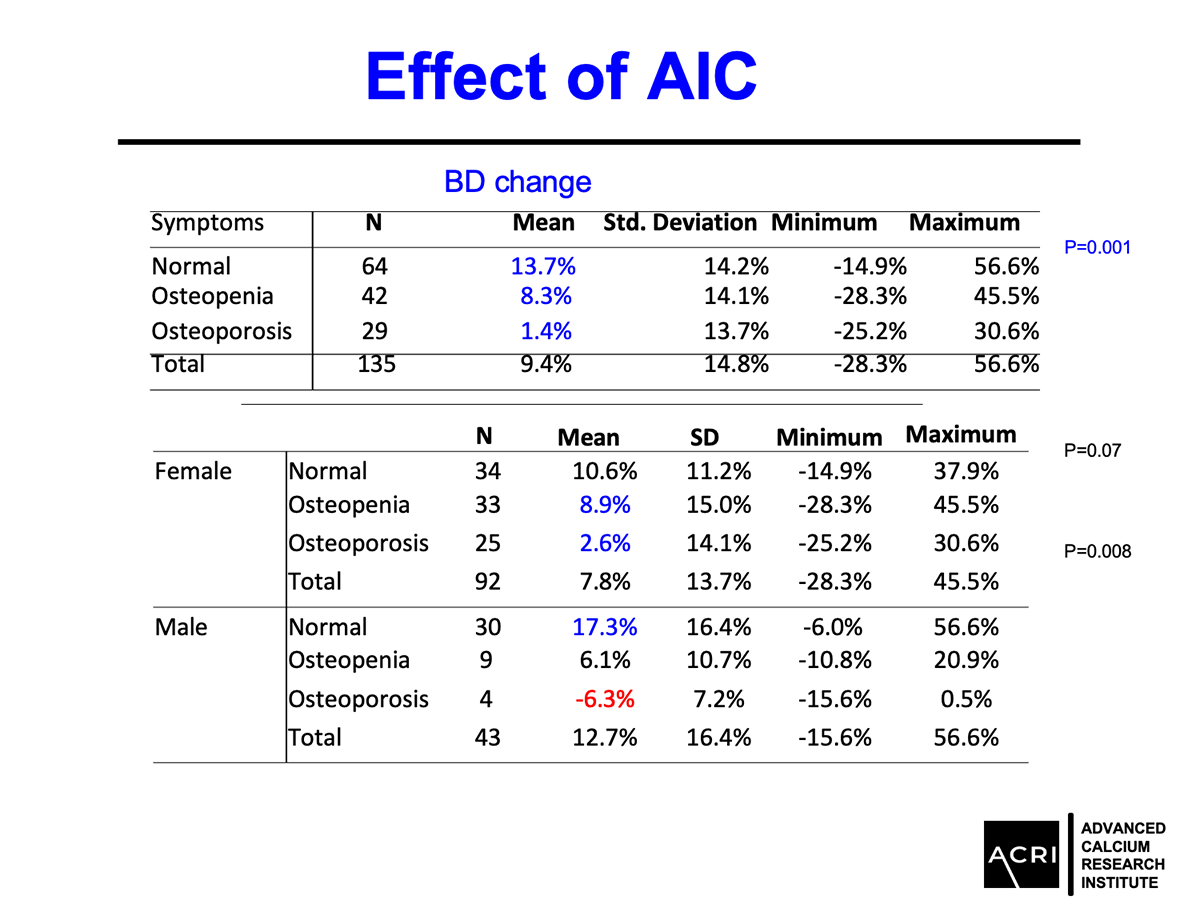
Mean bone mineral density increased by 9.4% across the entire cohort.
Patients with osteopenia and osteoporosis showed statistically significant improvements (p=0.0001).
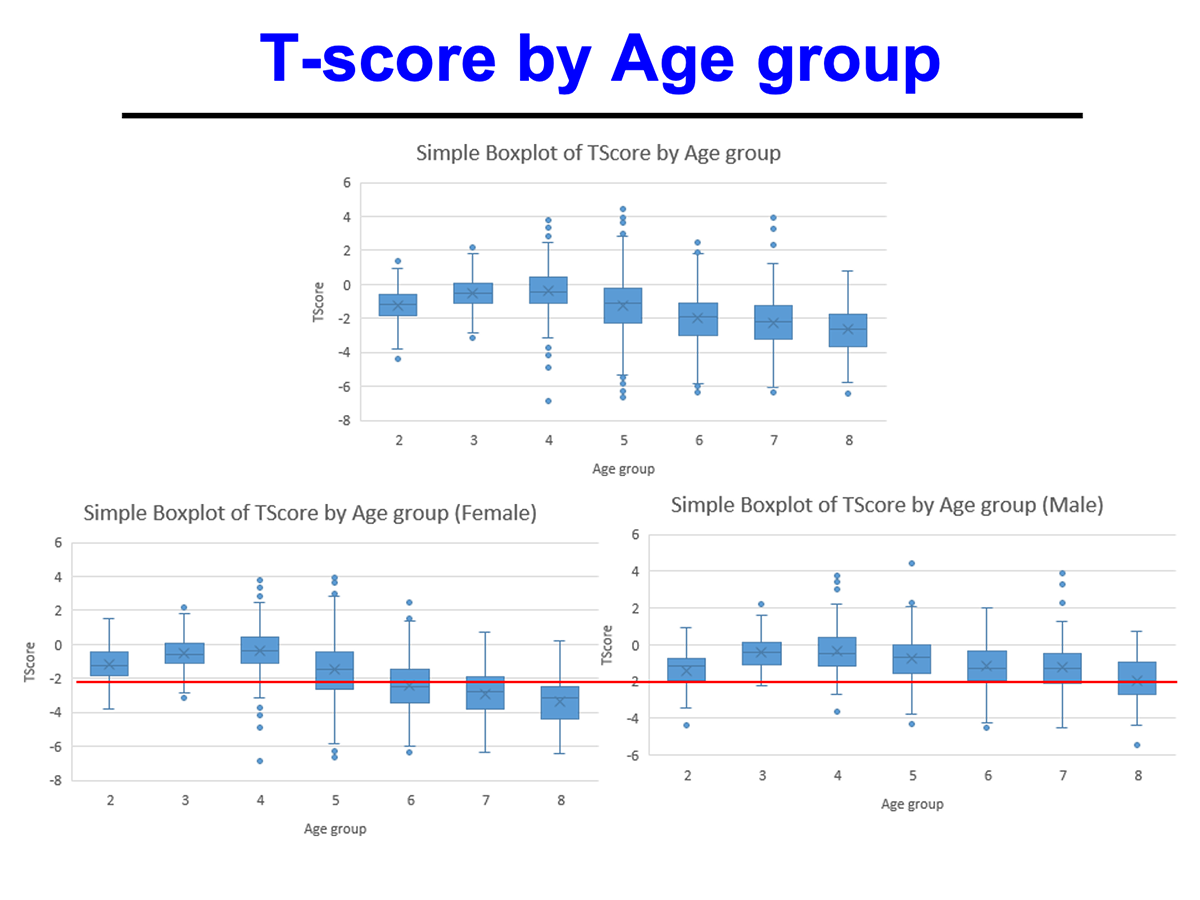
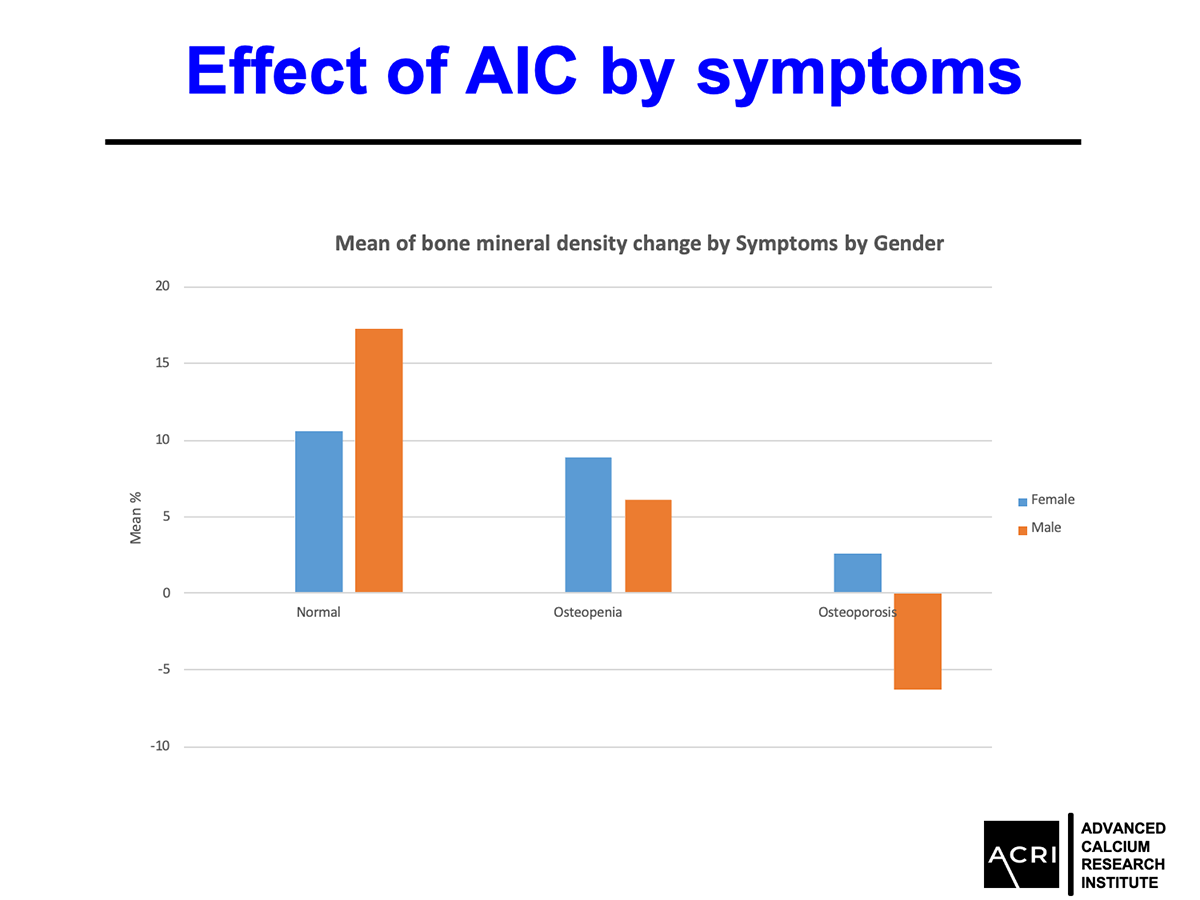
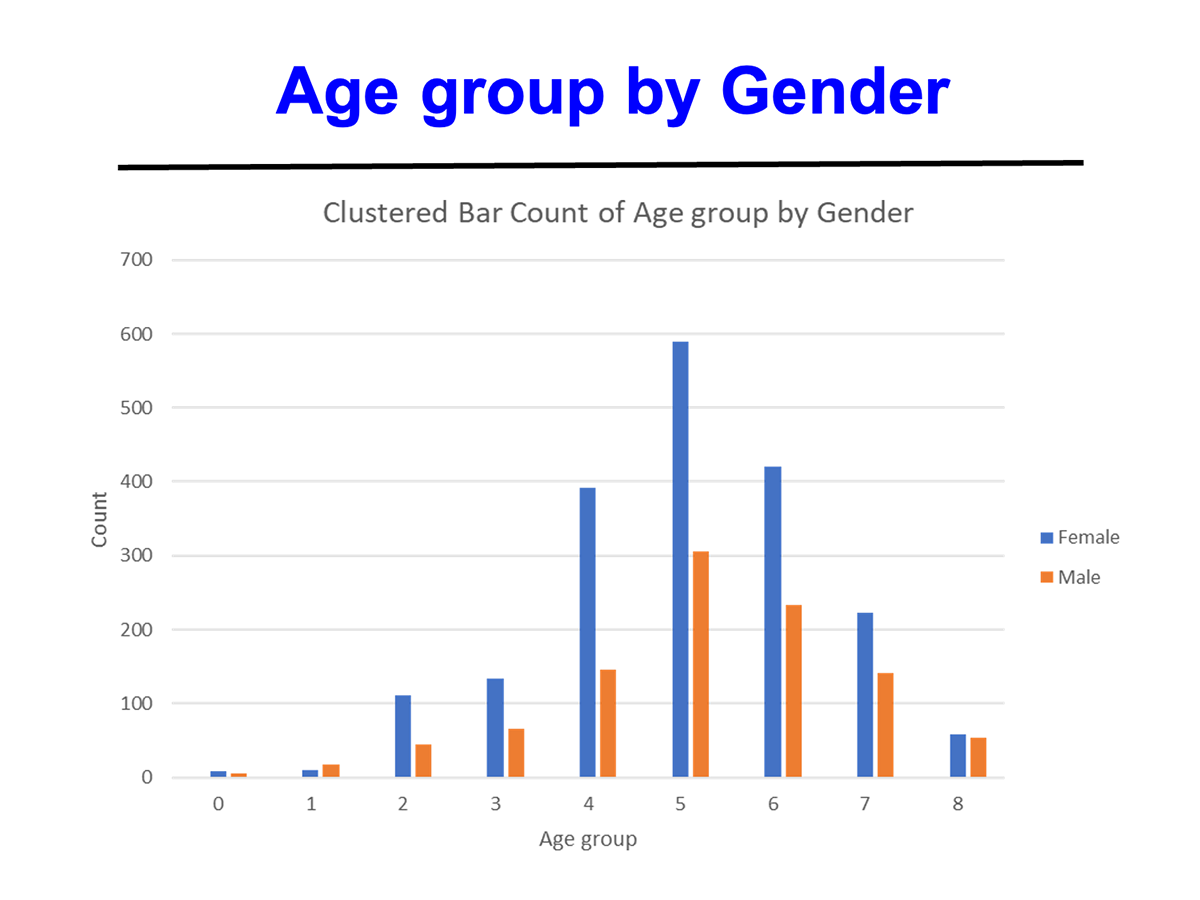
Male participants demonstrated greater average gains compared to female participants.
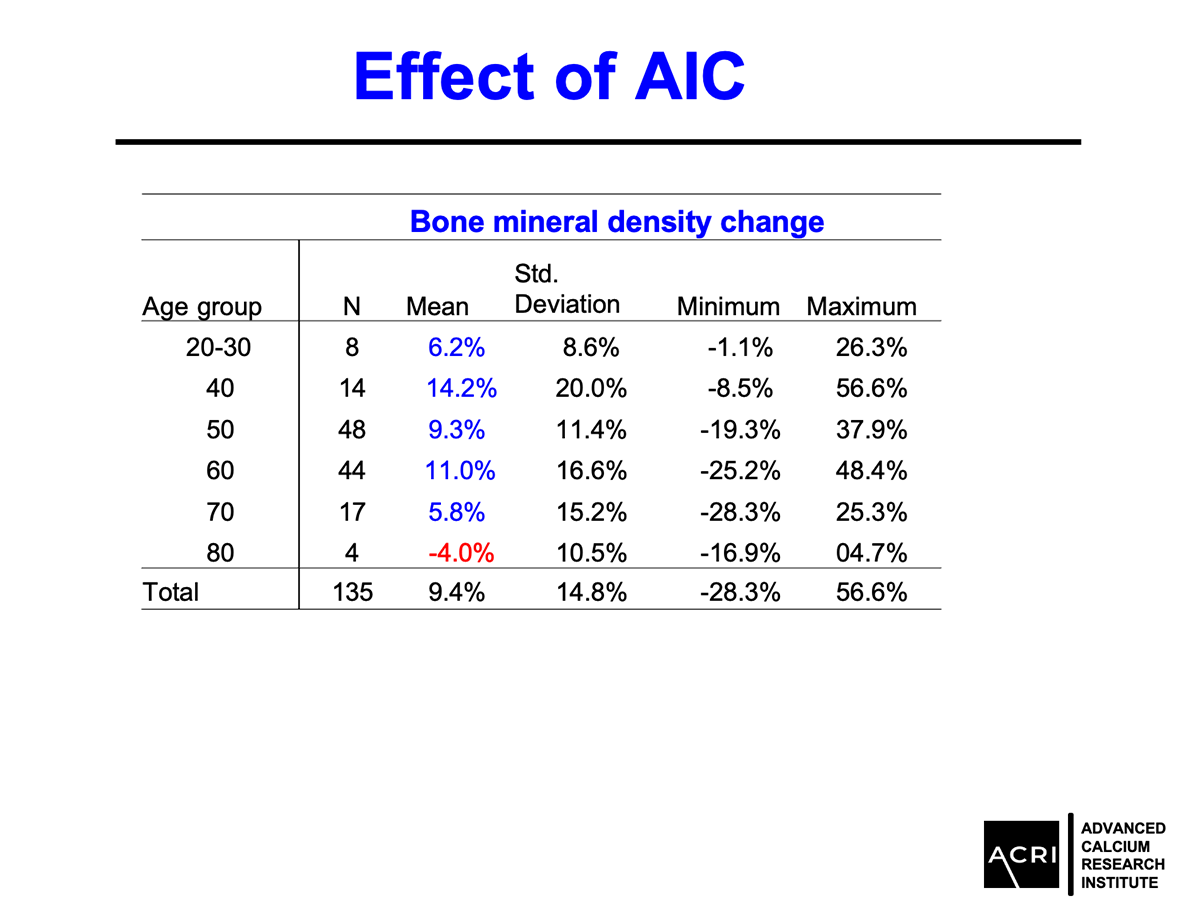
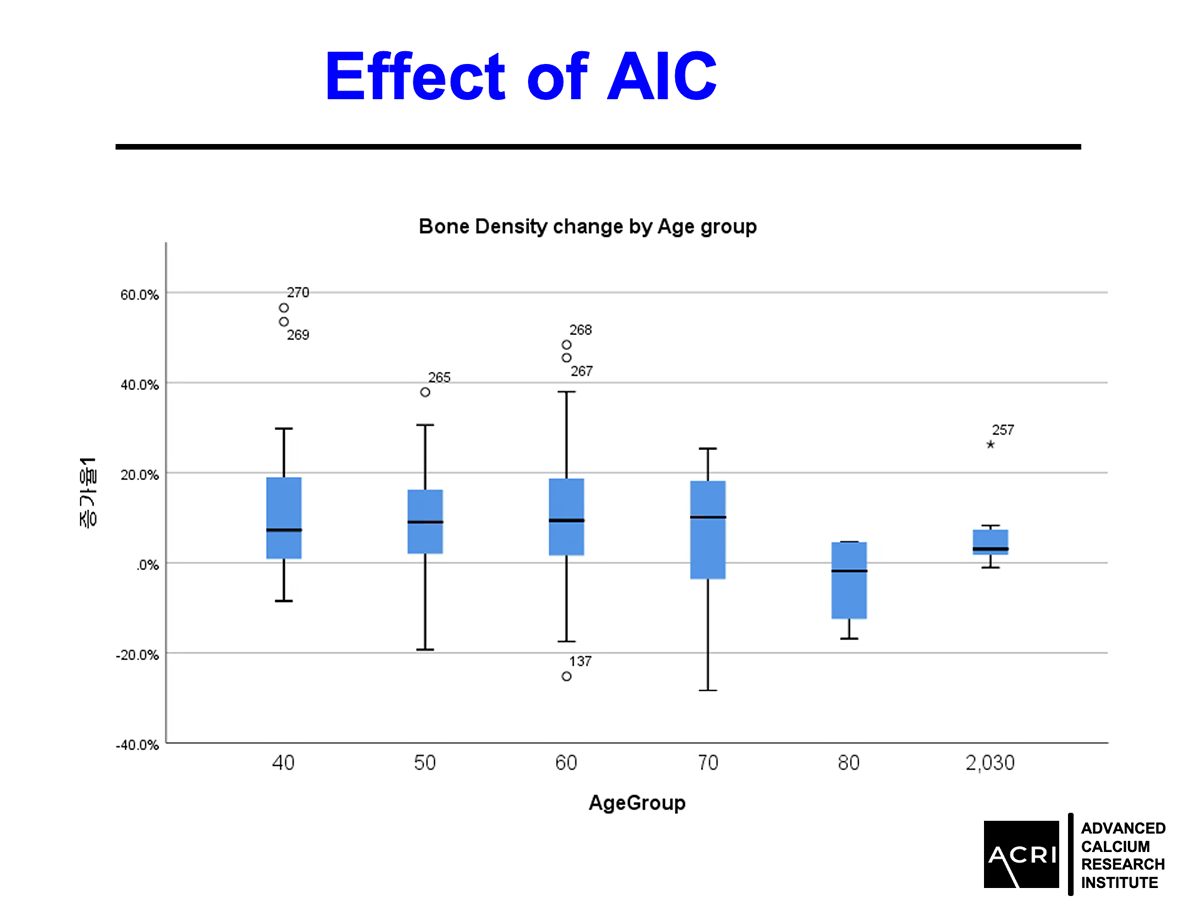
Improvements were observed across age groups and BMI categories, with overweight and obese participants showing the highest increases.
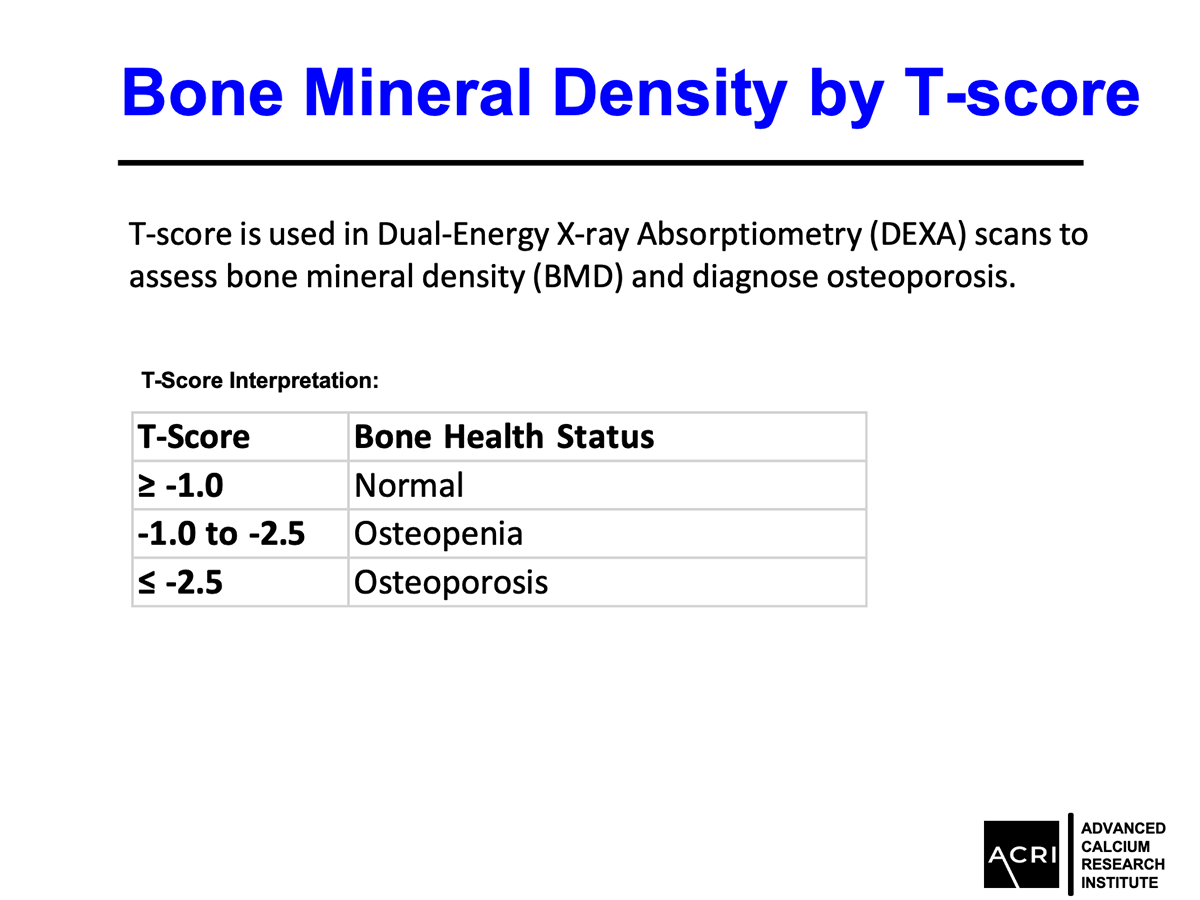
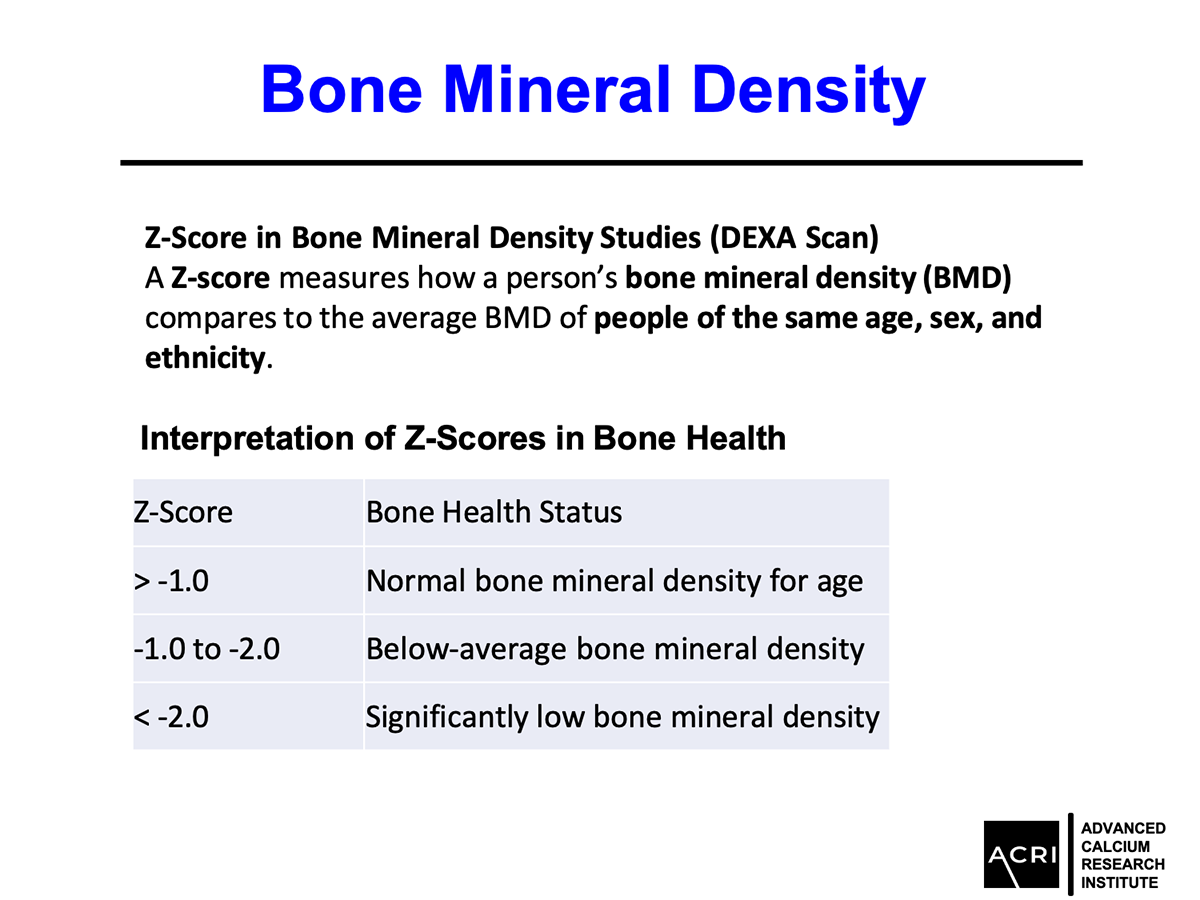
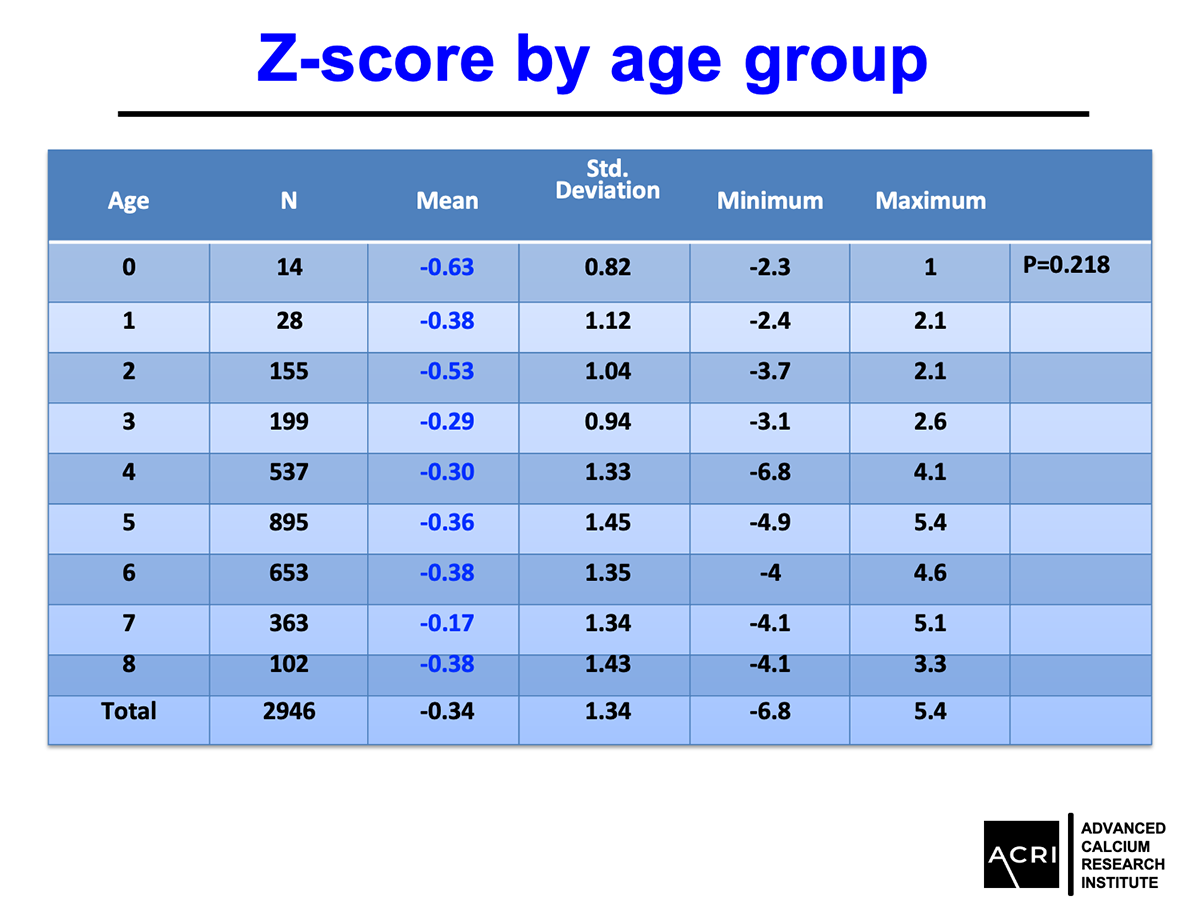
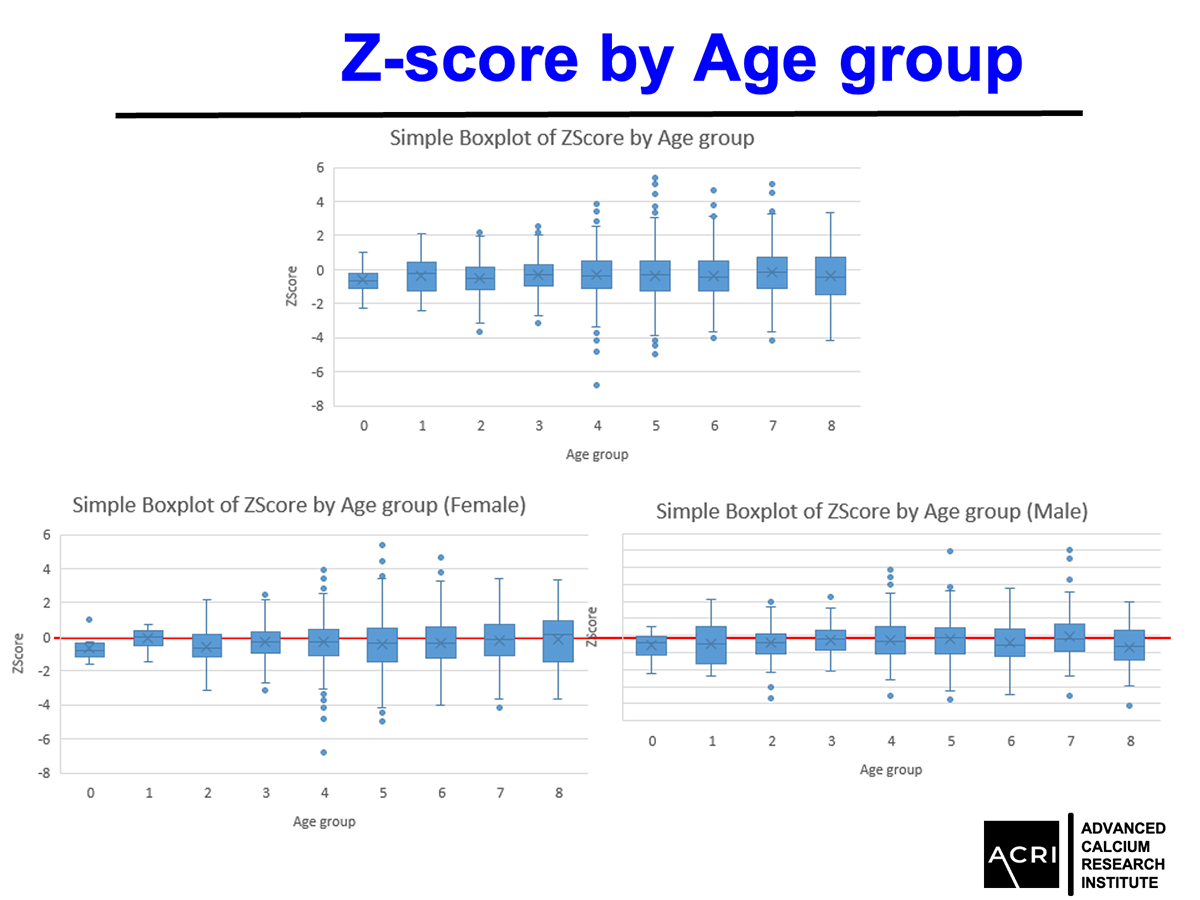
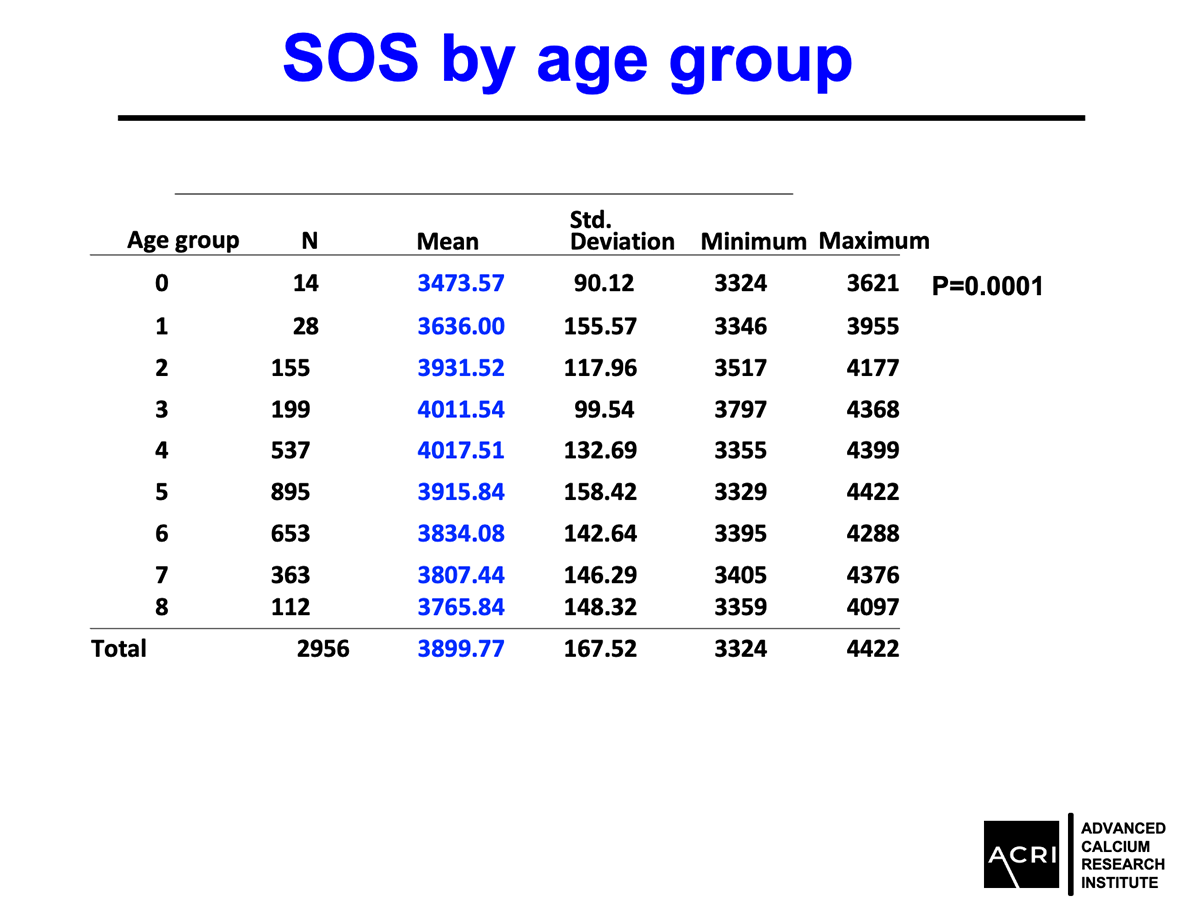
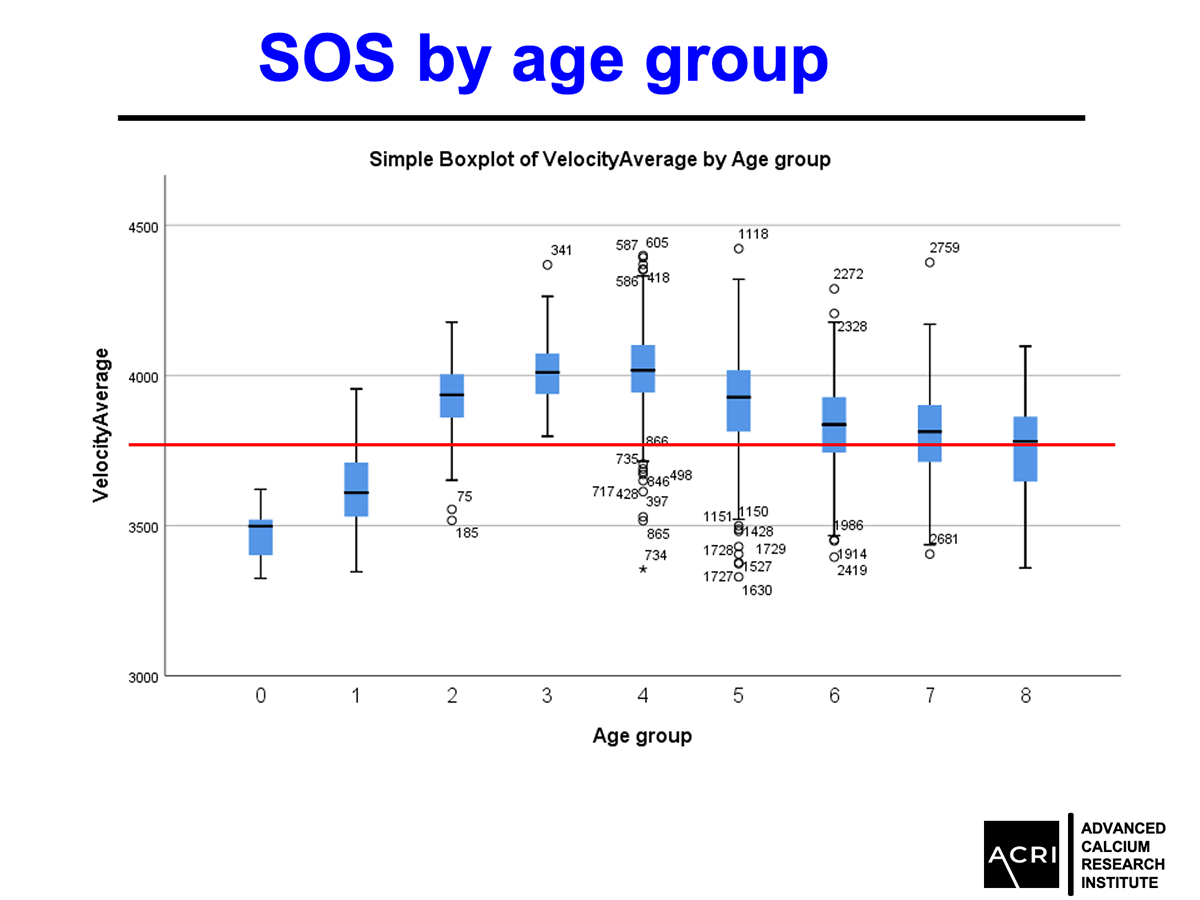
The T-score and Z-score distributions before and after AIC administration were analyzed using standard DEXA scans, and Speed of Sound (SOS) metrics further confirmed enhanced bone quality.
These findings support AIC calcium as a promising non-pharmacological intervention for bone health, with particular relevance to oncology patients, aging populations, and individuals with metabolic bone disorders.
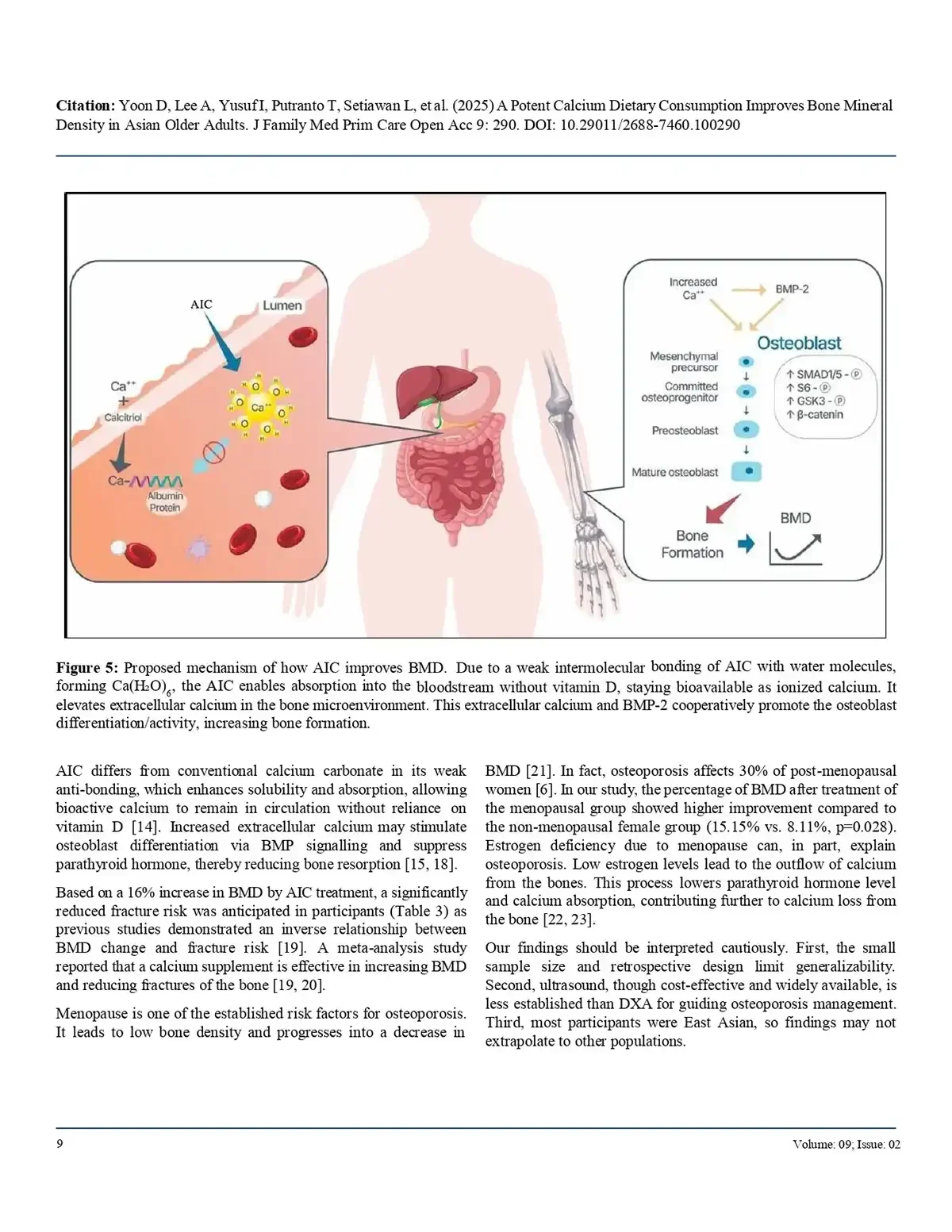
Anti-Orbital Ionic Calcium (AIC) demonstrates strong potential as a therapeutic agent for osteoporosis.
This study evaluates the protective effects of Anti-Orbital Ionic Calcium (AIC) on bone loss using an ovariectomy-induced (OVX) mouse model of postmenopausal osteoporosis. Over a 13-week oral administration period, AIC demonstrated significant benefits across multiple biological and structural indicators of bone health.
Researchers from Sungkyunkwan University and other institutions investigated the potential of a substance called AIC to mitigate osteoporosis using an ovariectomized (OVX) mouse model. The study demonstrated that AIC administration significantly improved bone mineral density and skeletal microstructure, effectively counteracting the bone loss typically caused by estrogen deficiency. On a cellular level, the treatment suppressed the expression of genes associated with osteoclast differentiation, such as TRAP and NFATc1, which are responsible for bone resorption. Furthermore, the researchers analyzed serum biochemical indicators and found that AIC helped stabilize markers of bone turnover without causing significant adverse effects on body weight or major organ health.
AIC treatment improved bone mineral density that had declined following OVX, indicating a restorative effect on skeletal strength. Serum biochemical markers—including bone alkaline phosphatase (BALP), osteocalcin, CTX, and P1NP—were favorably regulated, reflecting healthier bone turnover and reduced bone resorption. Gene expression analysis further showed that AIC suppressed key osteoclast-related genes, suggesting a molecular mechanism that limits excessive bone breakdown. Importantly, AIC did not cause adverse changes in overall body weight or major organ weights, supporting its safety profile in this model.
Overall, the findings highlight AIC as a promising therapeutic candidate for defending against bone density loss and improving osteoporosis outcomes, with benefits observed at both physiological and molecular levels.
AIC Supplementation Significantly Enhances Bone Mineral Density in an Asian Population: An Eight-Month Clinical Study
A recent clinical investigation demonstrates that Anti-Orbital Ionic Calcium Carbonate (AIC), a highly bioavailable form of calcium, significantly improves bone mineral density (BMD) in Asian older adults—a population known to have higher rates of osteoporosis and lower baseline calcium intake.
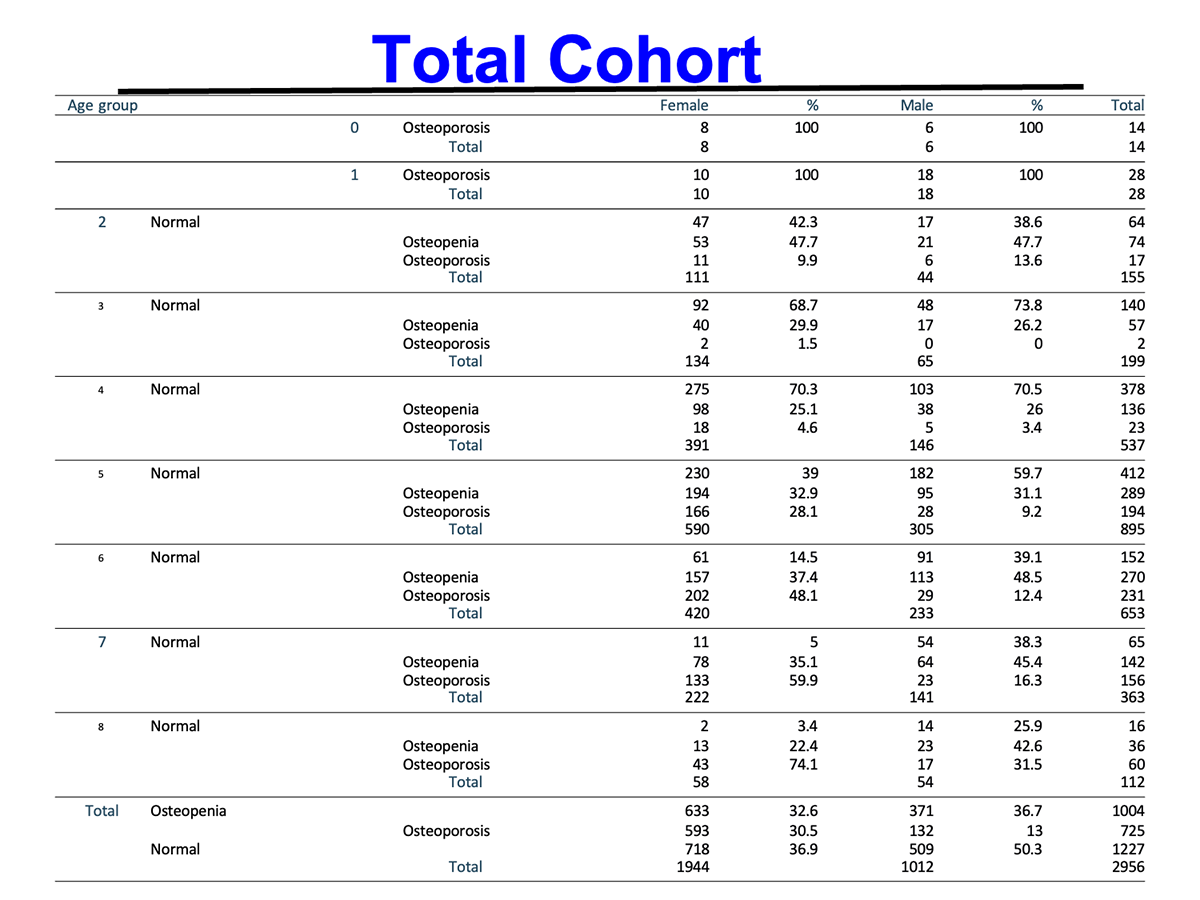
In this study of 82 participants, individuals were classified as normal, osteopenic, or osteoporotic and received daily AIC supplementation for approximately eight months. Bone health was assessed using ultrasound-derived metrics, including T-score, BMD, and speed of sound (SOS) at the distal radius.
AIC produced consistent and meaningful improvements across all groups:
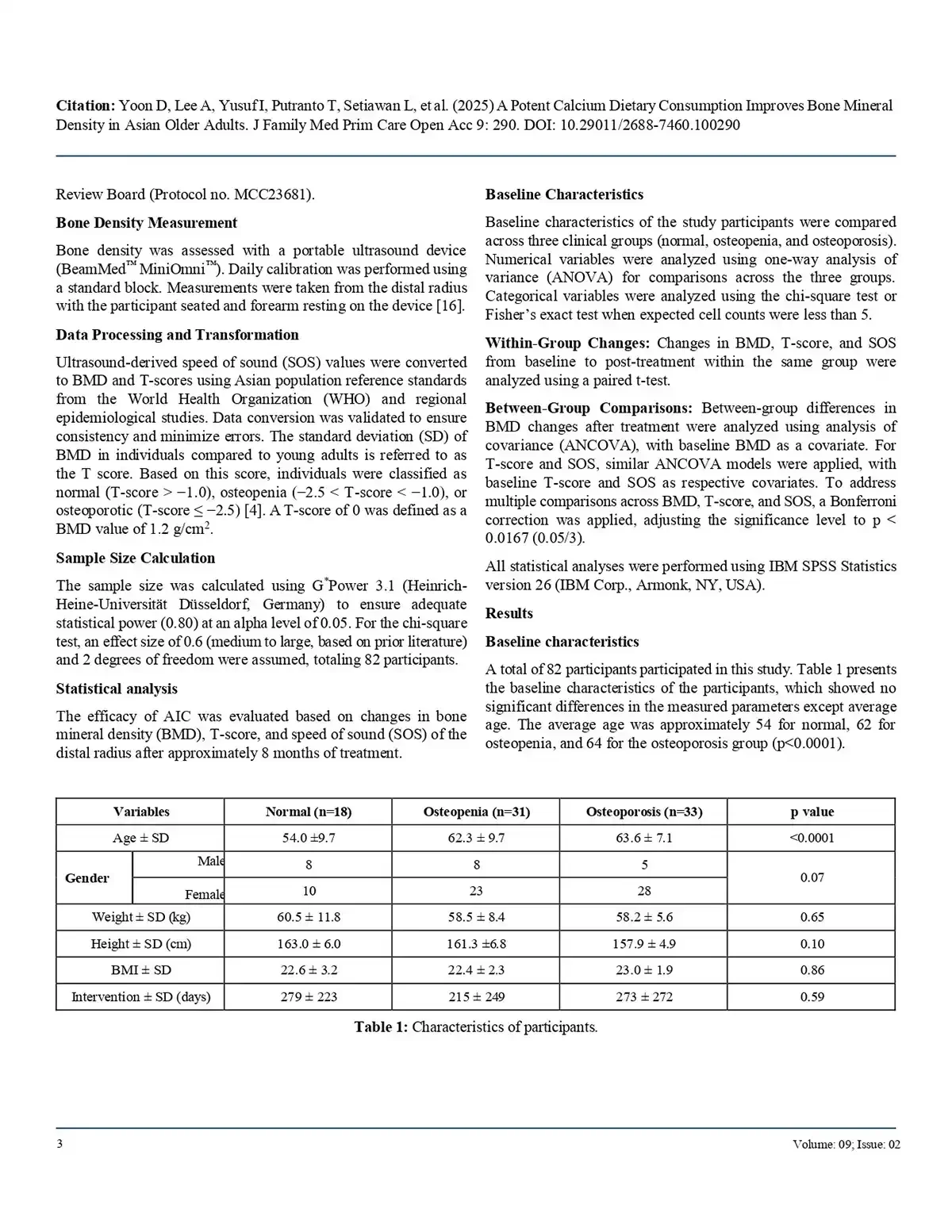
T-scores improved markedly (from –2.0 to –0.85, p<0.0001).
BMD increased significantly (0.95 to 1.10 g/cm², p<0.0001).
SOS values improved, indicating better bone quality (p<0.0001).
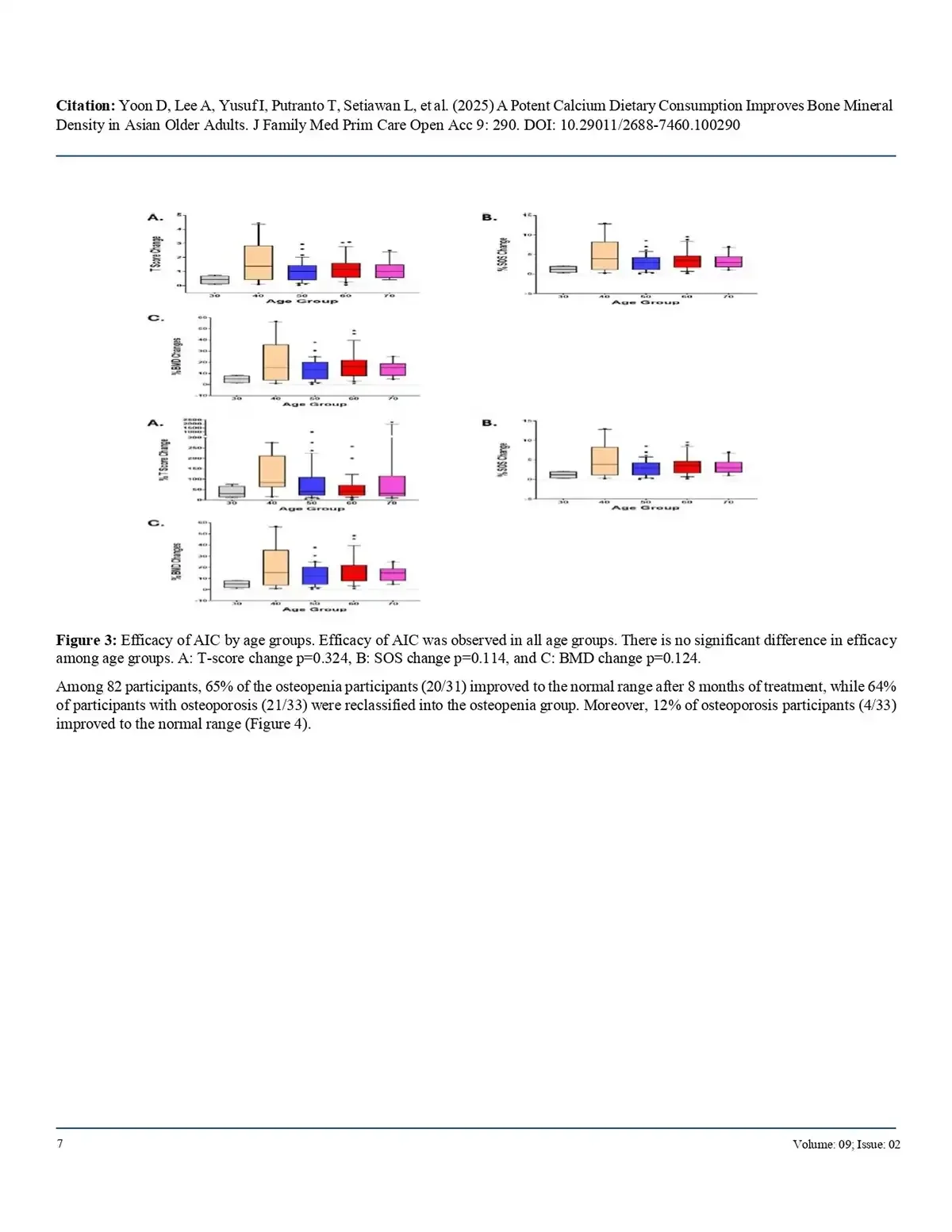
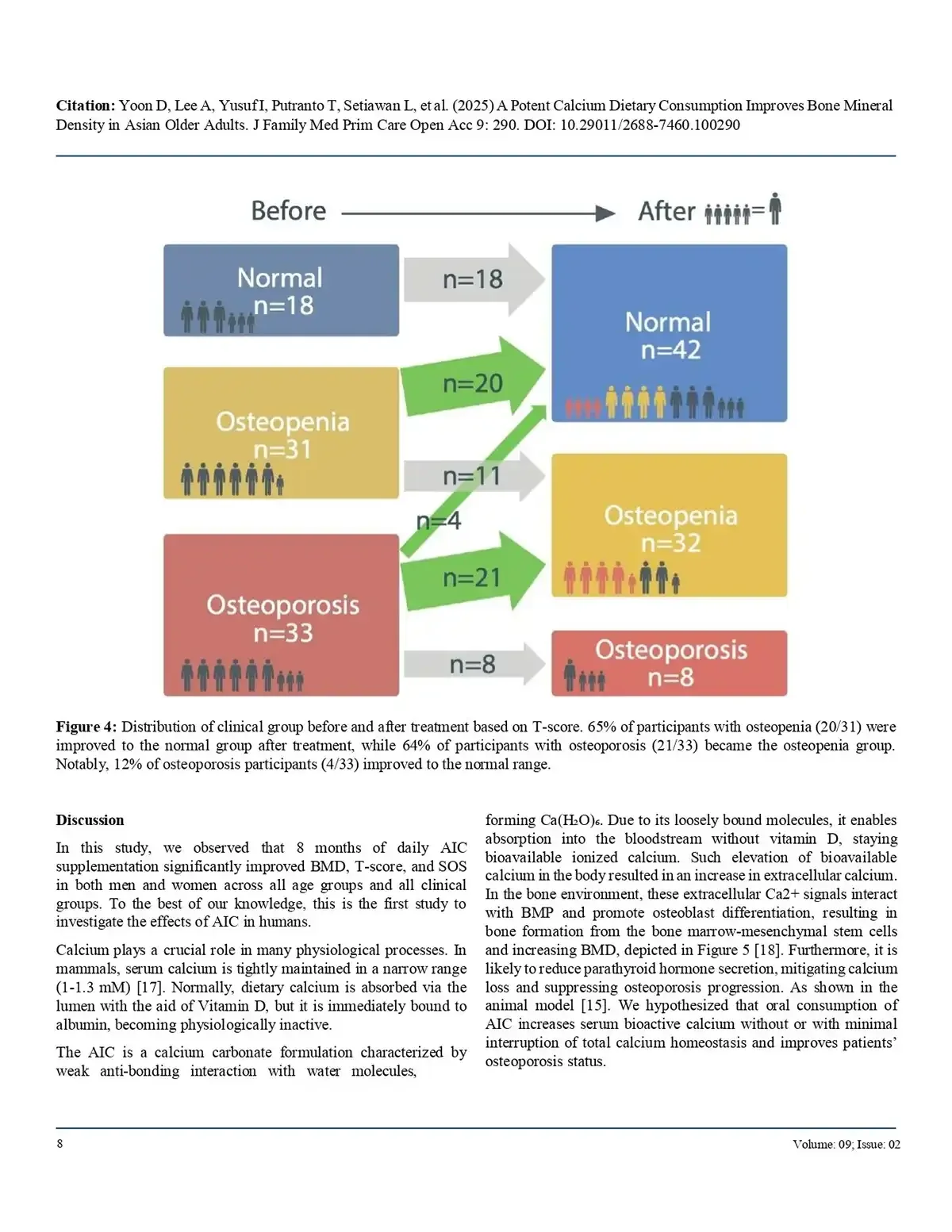
65% of osteopenic individuals returned to normal bone density, and
64% of osteoporotic participants improved to the osteopenic range.Notably, 12% of patients with osteoporosis achieved normal bone density.
These benefits were observed regardless of age or sex, with particularly strong effects in men and in participants with more severe baseline bone loss. Importantly, AIC demonstrated a favorable safety profile with no major adverse events reported.
Given its enhanced bioavailability and ability to support bone formation without reliance on vitamin D, AIC represents a promising nutritional strategy for improving bone strength and reducing fracture risk. Larger randomized trials are recommended, but current evidence supports AIC as a valuable option for individuals seeking to improve bone health and counteract osteoporosis progression.